Preparation of bromoethane
2010-03-06 17:37 by Ian
This is an attempt to brominate a primary alcohol.
Bromoethane was selected over bromomethane because bromomethane has a boiling point at about 3C. One possible side-product of this reaction would be dimethyl ether (BP = -23C). Ambient temperature is about 16C. EtBr would give a higher yield due to minimized loss to atmosphere. Also, all of these compounds are highly flammable. EtBr was deemed safer to make.
High-proof ethanol (distilled from cheap vodka) was used as the feedstock. NaBr was used as the source of the bromide ion and sulfuric acid as the catalyst.
Before large amounts of reagents were committed, a few test tubes were loaded with reactants and the best method decided by which tube had the lower boiling point. It turned out to be the tube on the left with the faint yellow tinge.

A fractional distillation setup was constructed and a separatory funnel was used as an addition funnel for a solution of NaBr.

Because this is a primary alcohol, the formation of a carbocation is not thermodynamically favorable. Upon addition of sulfuric acid to the ethanol, the OH group on ethanol is protonated to H2O+. This is a good leaving group, but not so good as to leave a carbon with a positive charge. What is likely to happen is the side-reaction of unreacted and protonated ethanol to form 1 mole of water and 1 mole of diethyl ether. Given the difficulty of separating the two, I added the acid to the ethanol slowly and with vigorous stirring to avoid causing local concentration and temperature spikes that might make the reaction favorable.
This reaction is SN2 and occurs at room temperature in a single step. Upon addition of NaBr, the freshly dissociated Br- ion attacks the carbon with a partial-positive charge. As the extra electrons rush into the carbon’s space, an electron is relinquished to the leaving group. The results are water, EtBr and NaHSO4.
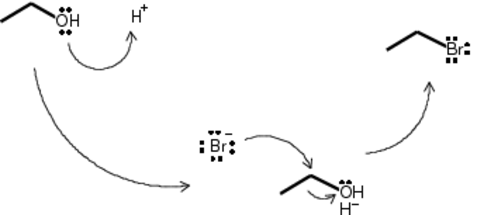
Because the EtBr is itself reactive, this process happens in reverse. In order to maximize the yield, the flask was warmed to the BP of EtBr to drive the reaction forward by lowering the concentration of EtBr in the reaction flask. Distillate was collected under water. Distillation proceeded at about 35C.

The chiller performed wonderfully. At about 70W, the coolant temperature held below 10C for the duration of the distillation.
Previous: Concentrating sulfuric acid (chiller test)
Next: Salesman in livechat