Extraction of ibuprofen from Advil tablets
2010-02-02 03:02 by Ian
After cleaning out a medicine cabinet in my house, I found a bottle of Advil tablets that was expired in 2007. As long as I was going to discard them, I decided to satisfy a curiosity about how they were constructed. This is my first attempt at isolation of ibuprofen from Advil gel-tabs.
Here is what I am trying to isolate:
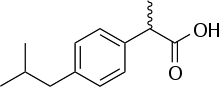
Notice: most of the molecule is made of C-H and C-C bonds. No wonder the solubility is poor… The squiggle line to the left of the carbonyl carbon indicates a racemic methyl group. This is the molecule’s only stereocenter, and in this case, it won’t affect the choice of extraction procedure. Both isomers (if both exist) will be extracted indiscriminately.
The general strategy is this…
I am willing to bet that converting this compound to a carboxylate salt with sodium as the cation will greatly improve its solubility in water. So I will basify, filter, acidify, filter again, and recrystallize the filtrate.
First, I have to destroy the gel coating so that the contents of the pill are exposed to the solution. In this case, water was used as the solvent. After the tablets sat in the water for about 24-hours, the container was agitated to break up the remaining tablets. A cloudy white suspension formed. According to Wikipedia, ibuprofen has poor solubility in water (less than 1mG per 1mL water). In order to actually get a yield, I had to get the ibuprofen into the aqueous solution.
I filter the gel coatings off the solution with a course filter and basify the solution with a few grams of NaOH and add a pH indicator. The solution is still murky, but it is now purple.

I shake the solution and filter off the solids, discarding them. The filtered solids are (I think) the binder that makes up the bulk of the pill’s mass. After filtration the solution looks much clearer. The solution was slowly acidified with dilute HCl and a white precipitate fell out of solution. As the pH approached neutral, less and less precipitate formed.
After lowering the pH of the carboxylate salt solution toward neutral, the equilibrium of the solution shifted to favor the carboxylic acid (which, due to its low solubility in water, falls out of solution as a precipitate). Filtration was performed to remove the pH indicator and the sodium chloride that formed as a result of my pH manipulation. After a wash with cold distilled water, sample was dissolved in warm acetone, recrystallized, and filtered again. A second wash with DI, a little heat, and the result is dry, isolated ibuprofen in the watch glass.

Like many other monocyclic benzene based compounds following work-up, this one looks like a brittle, foamy, low-density white powder. It has no taste.
This is the melting-point test that was used to determine purity and bolster confidence in the assertion that the isolated compound was indeed (RS)-2-(4-isobutylphenyl)propanoic acid. Pictured are two glass plates with a melted sample between them. Melting point agreed with literature.

This is the melting-point test after the sample was removed from heat. The ibuprofen is seen here in the process of recrystallizing to a solid.

The purified compound was then bottled, labeled and stored in the lab in case I got a really wicked headache. My friend Tim says hello. :-)

Previous: Take the Time
Next: Concentrating sulfuric acid (chiller test)