The problem with synthetic fats
2025-08-21 12:06 by Ian
Many people are (justifiably) skeptical of manufactured and GMO foods. So I answered some questions online for people, and it seemed to help enough bystanders that I felt it worth consolidating into an edited post.
Disclaimer: I am not being paid to write this, one way or another. It is not an endorsement of a company, nor a judgement on their value one way or another. Savor Foods is just the first I've heard of to do it, and so I will use their process as a use-case, but will refer to "food manufacturers" as a stand in for any company producing synthetic chemicals for mass-consumption.
A few background facts:
I won't assume the reader is a biochemist. So here are some basic facts taken for granted in this post.
- Animals store their energy in the form of carbon-hydrogen bonds.
- Carbon can hold four bonds to other atoms in a tetrahedral arrangement.
- Long chains of nothing but carbon and hydrogen are called fats.
- There are two basic kinds of fats: Saturated and unsaturated.
- The more unsaturated the fat, the less energy it contains.
- In biochemistry, shape is everything.
- The more unsaturated the fat, the greater the number of shapes it might take.
"[Un]Saturated":
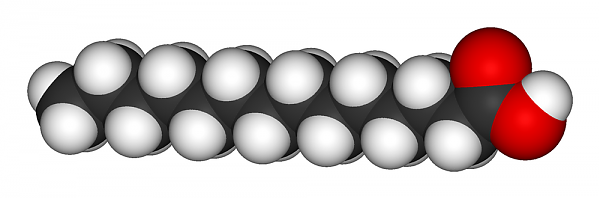
To say a fat is saturated means 100% saturated. All carbon-carbon bonds in the chain are single bonds, and there is no space on the fat molecule for additional hydrogen atoms without adding more carbon to hold it. There is only one possible shape for a saturated fat, and it looks like this:
There are varying degrees to which a fat might be unsaturated. We won't deal with this here, but just know that without a quantifier, "unsaturated" means that there is at least one carbon-carbon bond that is not single, and therefore a potential bond site for two additional hydrogens (one per carbon atom). But it might also mean many unsaturated sites.
"Transfat"
A "transfat" is a specific kind of unsaturated fat. But not all unsaturated fats are transfats. If an unsaturated fat is not a transfat, it is a cisfat. Here is an example with a single unsaturation:
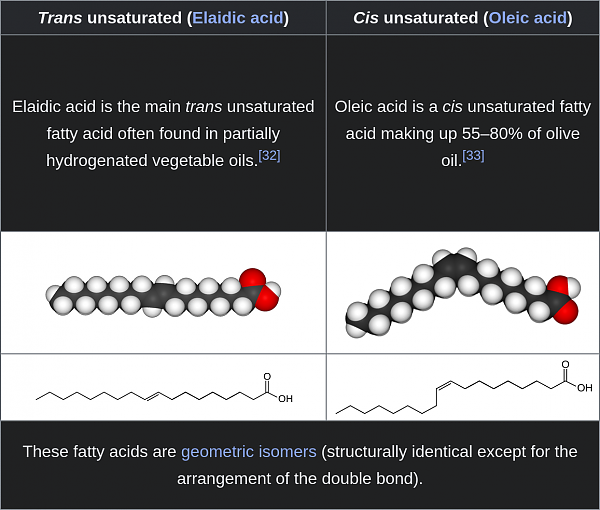
Cis bonds are less-stable than trans bonds, all else equal. But not usually so much so that normal conditions for life would convert them. However, if a conversion were to happen, cis ---> trans is much more likely than trans ---> cis. Mostly because a trans bond continues in a straight line as a saturated fat would do, and therefore carries lower potential for steric strain.
When you tell a chemist or biologist "transfat", they will picture an unsaturated fat with no cis bonds.
Why would you want to make fats synthetically?
There are at least these three motives, ranked by how common they are:
- Co$t: Higher energy density costs more in Nature. And that means it will co$t more. So if you can "enrich" a fat, you can give it qualities that our brains and bodies are adapted for appreciating. Butter is more appealing than oil. And cows are slow and inefficient, whereas plants (which yield mostly unsaturated fats) scale comparably well.
- Tuning: Because fats are energy storage for animals, it might be useful to decouple (the fat's shape) from (its energy content). Since shape gives rise to bulk material properties, you could make something that resembled butter, but had a fraction of the calories. Or make an oil that was a precursor to something for which a patient was deficient. And you might want to do such things even if it increases co$t.
- Creating fats in places where there is no agriculture. Think: moon bases and spacecraft.
The problem with making fat from CO2 is twofold:
1) The energy cost is tremendous. I wrote a run-down of the thermodynamics of reducing that much CO2 in service of a different question, but the same applies here. There is precious little oxygen in a fatty acid, and because most of the energy cost is pulling oxygen off the carbon, so the energy cost for reduced carbon should be comparable for a fully-synthetic fat on a per-carbon basis. The process would be much cheaper to feed with methane. But let's consider this a side-issue which might be solvable by other means. This post is for biochem, not enginerding.
2) Possibly all of the same problems as transfats. If the fats are not created by enzyme action, the saturation/unsaturation pattern in each fat chain will not be predictable, nor will be its chirality. You will get a complex mix of shapes. And since shape is nearly everything in biochemistry, you will wind up with many deposits of "half-eaten" fats in the tissues that metabolize or transport them.
Q&A:
Q: What differentiates a synthetic CH chain from a natural one?
There isn't a difference, potentially. A CH chain produced by a cow MIGHT be exactly the same as one made by whatever Frankenstein microbe they've made to do the same task.
If the fat is fully-saturated, as a cow would probably produce, there is no room for differences in anything but the LENGTH of the fat chain.
Fully-saturated fats have higher freezing points (butter is a solid at room temperature instead of a liquid oil), they have higher energy density, and they taste sweet to us. Butter is made by removing water from milk and concentrating the saturated fats until the product is solid. But it still contains some unsaturated fat.
Consider these two unsaturated fats: Linoleic acid (on the left), and Linolelaidic acid (on the right)
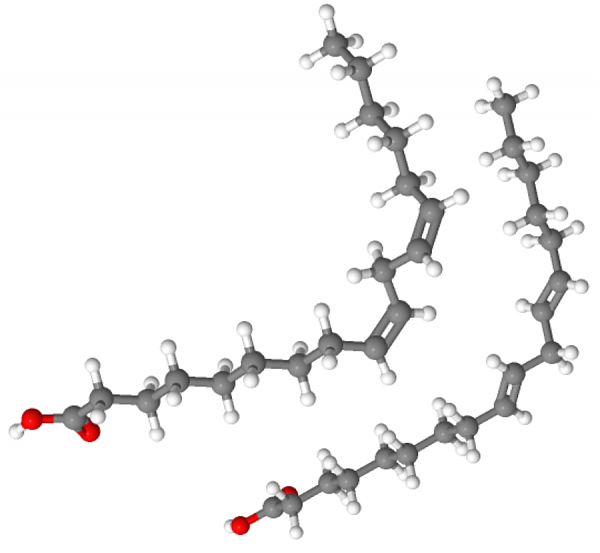
That "kink" in the middle of the chain prevents the fish from freezing solid in the cold ocean, at the cost of the slightly higher energy content of fat made by a warm-blooded terrestrial animal. But the molecule is not free to rotate wherever there are C-C double bonds, so unsaturated fats have the potential to be not only left-or-right handed (chiral isometry), but also take on one of two different conformations (geometric isometry). And this makes the biochemistry far more complicated.
You must eat the thing on the left as an essential nutrient. But eating the thing on the right appears to give people heart disease. Maybe your body will handle linoleic acid just fine, but you don't have an enzyme that fits the shape of the linolelaidic acid. And, unable to break the linolelaidic acid apart at reasonable rate, it (and its carrier) builds up in your arteries. The water solubility of most fats (even fatty acids) is very poor, so if you don't have the enzymes for it, it will just bioaccumulate. Or maybe your body tries to use the mutant fat as raw materials to make a cholesterol or steroid. But the mutant fat results in a product that is the wrong shape (same isomer problem, but in a metabolite molecule).
This is the reason transfats are bad for your health, long-term. Your body will likely be able to eat any unsaturated fat Nature produces (including natural transfats), having co-evolved with the organisms you are eating. Just an idle hypothesis on my part, but the point is: those unsatuated fatty acids only differ in their unsaturated bonds, and that is difference between health-giving food, and poison.
So as a traditional chemist making the Frankenbutter via near-total synthesis, the trouble is that the "kink" might happen potentially anywhere in the chain (or be facing the wrong way), and still weigh the same as a fat with a correct kink in the correct place. You may wind up with a fraction of fat being unsaturated, and you will either need to tolerate it, or expend energy and yield to purify it.
To my knowledge, the most common synthetic fats are made by catalyzed reduction of carbon-carbon bonds by hydrogen gas under high pressure and heat. Enough pressure and heat such that the natural cis bonds in the fat are spontaneously converted to trans bonds ahead of being reduced by hydrogen.
But nothing in chemistry is 100%. For the sake of bulk material properties and taste, "food" manufacturers (I don't consider transfats food) consider a certain percentage of unsaturation to be optimal. Purification and hydrogen are both expen$ive, and a transfat has the desired higher melting-point with less biochemical energy (it is still unsaturated). Since the fats were held above the conditions of converting cis ---> trans, the fat has been denatured with practically all of its cis bonds reduced or made trans. Hence, the label by which transfats are called.
Q: Are there any inherent difficulties in synthesizing lipids that are more severe than other, more complex organic molecules?
From my knowledge, saturated fatty acids and the macromolecules they are commonly a part of should be the easist biomolecules to make synthetically. You can almost dig the complete molecule out of any number of things. Paraffin wax is just a saturated fat with carbon chains in excess of 48(?) carbons long. If you were to crack and acidify paraffin, and then react the result with glycerine, you've made edible triglycerides from candle wax and a special kind of alcohol. At probably a very good yield.
Sugars (and unsaturated fats) are much more difficult, due to their chiral and/or cis-trans isomerism. Either kind of isomerism leads to the requirement of either.....
A) Filtering away all of the stuff you don't want from the results. Which (all else equal for ingredients and conditions) would be approximately half your yield.
B) Finding a chemical reaction that is highly selective, and only makes exactly the change you want.
This is why biology is good at chemistry, and we suck at it. We can't usually take option (B), because our chemistry praxis and knowledge only lets us adjust bulk conditions. We can't grab individual molecules and only stick this specific hand-chosen thing on that specific place. This is why chemists might consider a yield of 3% to be an excellent result for a complicated synthesis. They might need to lose half their yield at many steps.
Most drug makers don't even bother filtering for chiral racemates. When you take a drug that has a chiral isomer, you probably take twice the needed amount on the presumption that half the drug will be biologically inactive and be removed without consequences.
You hope...
I won't even consider proteins, since those are so complicated and fragile, that humans can't make them by traditional chemistry praxis. It requires biology (which is naturally-evolved nanotechnology, for our purposes here). Humans never make proteins with chemistry directly. We modify something like E. coli or a yeast to do it for us, and then harvest a crop from cultures that are grown in incubation vats. To do as Life does, and make chemistry a per-molecule affair is a (depending on who you ask) literal act of God.
Conclusion
I would consider eating the result of this synthesis on two conditions:
1. I could be assured that the anabolic phase of the synthesis ("building up" from C+H2, and CH4) produced 0.0% unsaturated products as a thermodynamic certainty. No unstaurated fats ---> No transfats
2. The post-cracking weight distribution of the products in what the company considers "acceptable yield", or otherwise, their measures for filtering out the long stuff like paraffin wax (which my body won't process or be able to eliminate). If their methods preclude production of hydrocarbons above a certain length, then this assurance might not even be necessary.
If those two things are known and controlled for, I think there would be practically no difference between what was synthesized, and the analogous molecules you would eat from a cow. The cow, however, would also give you other nutrients and flavors. Some of which we don't know we need because (having drank cow milk our whole lives), we take their benefits for granted.
"Food" manufacturers make me think of this kid at the science fair, and I wonder if that kid grew up and now has a C-level job in the food division of some billion dollar chemical company.
Would I eat their sawdust? If the process is good, honest, and maintained? Sure.
But compared to butter, it'll still be akin to eating sawdust.
The real magic of this synthesis would be in the anabolic phase, not in their cracking. If they have really made a large energy leap in making fatty acids from methane, if would be impressive, and they deserve to succeed, IMO. My suspicion is that in practice, they will feed a bioengineered yeast culture from cow farts to basically produce oil. If you could make a yeast that turned fermenting cow shit (or your shit) into oil that you could then use for crafting and fuel, even if you never use it for food. And if that yeast behaved like the yeasts we all know and love (and whose waste we happily eat), then that seems to me like a perfectly acceptable tech to have.
You probably don't want to get rid of the cow, since you still need methane. You would just stop wasting their farts, and get a bottle of (machine lubricant), or (animal feed enhancer), or (engine fuel) every month.
I think the idea of making food out of it is infeasible. They say as much in their statement: "with the potential to achieve cost parity with commodity oils in the longer term."
So it's more expensive than hydrogenated seed oils. If the economics aren't there, it won't end up in food.
Unless it gets deployed in space, or an underground bunker.

Previous: The problem with climate modeling
Next:
